Adipocyte derived glutathione promotes obesity-related breast cancer
Adipocyte-derived glutathione promotes obesityrelated breast cancer by regulating the SCARB2ARF1-mTORC1 complex
Chenxi Zhao, Tingting Zhang, Si-tu Xue, Peitao Zhang, Feng Wang, Yunxuan Li, Ying Liu, Luyao Zhao, Jie Wu, Yechao Yan, Xiaoyun Mao, Yuping Chen, Jian Yuan, Zhuorong Li, and Ke Li
Cell Metabolism (IF 27.7) Pubdate:2024.10.18
DOI:org/10.1016/j.cmet.2024.09.013
Abstract:
Obesity is a major risk factor for poor breast cancer outcomes, but the impact of obesity-induced tumor microenvironment (TME) metabolites on breast cancer growth and metastasis remains unclear. Here, we performed TME metabolomic analysis in high-fat diet (HFD) mouse models and found that glutathione (GSH) levels were elevated in the TME of obesity-accelerated breast cancer.
The deletion of glutamate-cysteine ligase catalytic subunit (GCLC), the rate-limiting enzyme in GSH biosynthesis, in adipocytes but not tumor cells reduced obesity-related tumor progression. Mechanistically, we identified that GSH entered tumor cells and directly bound to lysosomal integral membrane protein-2 (scavenger receptor class B, member 2[SCARB2]), interfering with the interaction between its N and C termini.
This, in turn, recruited mTORC1 to lysosomes through ARF1, leading to the activation of mTOR signaling. Overall, we demonstrated that GSH links obesity and breast cancer progression by acting as an activator of mTOR signaling. Targeting the GSH/SCARB2/mTOR axis could benefit breast cancer patients with obesity.
1. Research background
Obesity is a major risk factor for breast cancer and is associated with a poorer prognosis for breast cancer.
Obesity alters the metabolism of the tumor microenvironment (TME), but how obesity-induced TME metabolites affect breast cancer growth and metastasis has been unclear.
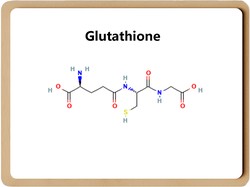
Glutathione (GSH) is the most abundant metabolite in cells, and its elevated level is associated with tumourgenesis, development, and drug resistance.
Inositol protein kinase (mTOR) is a metabolite sensing and signaling pathway whose activation is correlated with amino acid levels.
Cholesterol esterase B2 (SCARB2) is a transmembrane glycoprotein whose function depends on its interacting ligand.
2. Research results
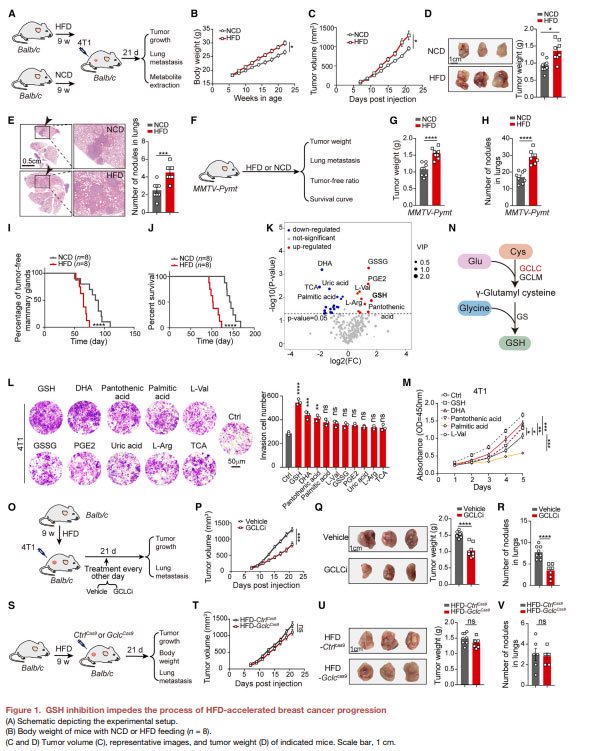
The progression of obesity-related breast cancer is closely related to glutathione secreted by fat cells.
In obese mouse models, breast cancer tumors were larger in size and weight and had more lung metastases, and increased glutathione levels were a key factor.
The expression of glutathione synthetase (GCLC) and the level of glutathione were increased in adipocytes, and exogenous glutathione could reverse the inhibitory effect of adipocyte Gclc knockout on tumor progression.
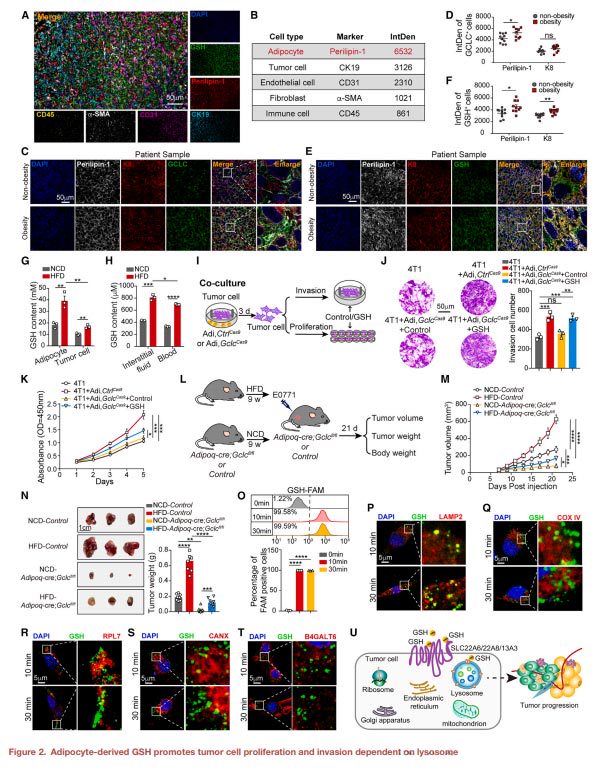
Glutathione binds to the lysosomal membrane protein SCARB2 and interferes with the interaction between its N and C terminals, thereby activating the mTORC1 signaling pathway and promoting the growth and metastasis of breast cancer cells.
SCARB2 knockout can inhibit glutathione-mediated breast cancer cell progression, and SCARB2 knockout can reduce breast cancer progression in obese mice.
ARF1 plays a key role in glutathione-mediated mTORC1 signaling pathway activation, and ARF1 knockout can block the tumor-promoting effect of glutathione.
mLST8 is a key subunit in the mTORC1 signaling pathway, and its down-regulation can inhibit glutathione-mediated progression of breast cancer cells.
Inhibition of glutathione production, SCARB2 expression, or ARF1 activity may help mitigate the progression of obesity-associated breast cancer, providing new ideas for the development of therapies targeting obese breast cancer patients.
3. Research significance
The authors reveal the critical role of glutathione in the progression of obesity-associated breast cancer and the interaction between glutathione, SCARB2, and ARF1, namely that inhibition of glutathione production, SCARB2 expression, or ARF1 activity may help mitigate the progression of obesity-associated breast cancer.
This study provides new ideas for the development of therapies for obese breast cancer patients, particularly targeting the glutathione /SCARB2/mTOR axis, but further research is needed to explore the prognostic and therapeutic significance of this pathway in obese and non-obese breast cancer patients.