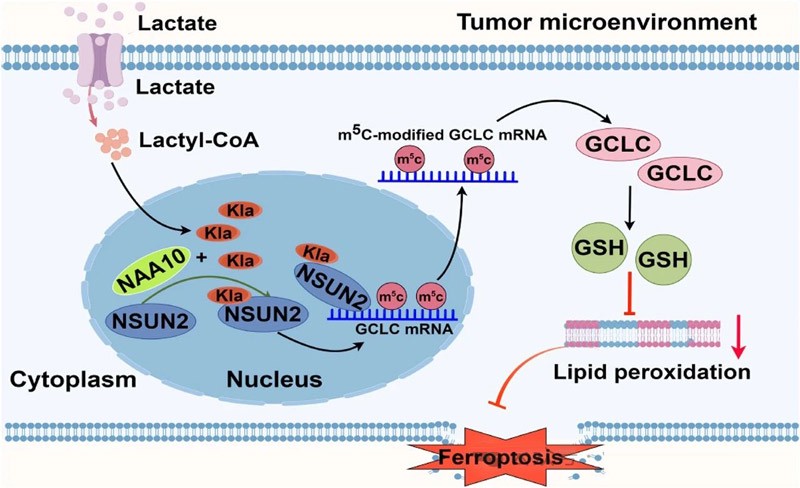
Enhancing GCLC-dependent glutathione synthesis to drive cancer cell resistance to iron death
Molecular mechanisms of genomic variation and tumor progression, mitochondrial homeostasis maintenance and neurodegenerative diseases.
The article is entitled "NSUN2 Lactating drives cancer cell resistance to ferroptosis through enhancing GCLC-dependent glutathione. synthesis ".

The aim was to explore the mechanism of how lactation of NSUN2 enhances GCLC-dependent glutathione synthesis and thus enhances cell resistance to doxorucin (Dox) -induced iron death, and to focus on key words such as NSUN2, lactation, RNA 5-methylcytosine, GCLC, glutathione synthesis, and iron death.
backdrop
Lactic acid-mediated lactation modification is a newly discovered post-translational modification that has important effects on protein function.
The 5-methylcytosine (m5C) modification in RNA is dynamic and reversible, suggesting that the activity of its methyltransferase NSUN2 may be regulated.
How NSUN2 activity responds to acidic conditions in the tumor microenvironment and how it regulates cancer cell survival is unclear.
aim
The aim of this study was to explore how NSUN2 activity is enhanced by lactate-mediated lactation modification and how this process promotes GCLC m5C formation and mRNA stabilization by targeting glutamate-cysteine ligase catalytic subunit (GCLC) mRNA, thereby affecting the resistance of gastric cancer cells to iron death.
method
The NSUN2-knockout MKN45 cell line was constructed by CRISPR/Cas9 technology, and the target cell clones were obtained by flow cytometry.
The proteins interacting with NSUN2 were identified by co-immunoprecipitation (co-IP) combined with liquid chromatography-mass spectrometry (LC-MS/MS).
Non-targeted metabolomics analyses were performed to compare metabolic changes between NSUN2 wild-type and knockout MKN45 cells.
result
We found that lactate activates NSUN2 by enhancing the interaction between NSUN2 and NAA10, which in turn promotes m5C modification and stabilization of GCLC mRNA, resulting in increased intracellular glutathione (GSH) levels and reduced lipid peroxidation, thereby making gastric cancer cells resistant to doxorubicin (Dox) -induced iron death.
It was also found that the K508 site of NSUN2 is the main lactate site, and NAA10 is the lactate transferase of NSUN2.
conclusion
This study revealed that NSUN2 lactation promotes cancer cell survival by enhancing GCLC-dependent glutathione synthesis.
Lactate treatment significantly enhanced the interaction between NSUN2 and lactoacyltransferase NAA10, resulting in enhanced lactation and enzyme activation of NSUN2, which then targeted GCLC mRNA to promote m5C formation and mRNA stability, thereby inducing GSH synthesis.
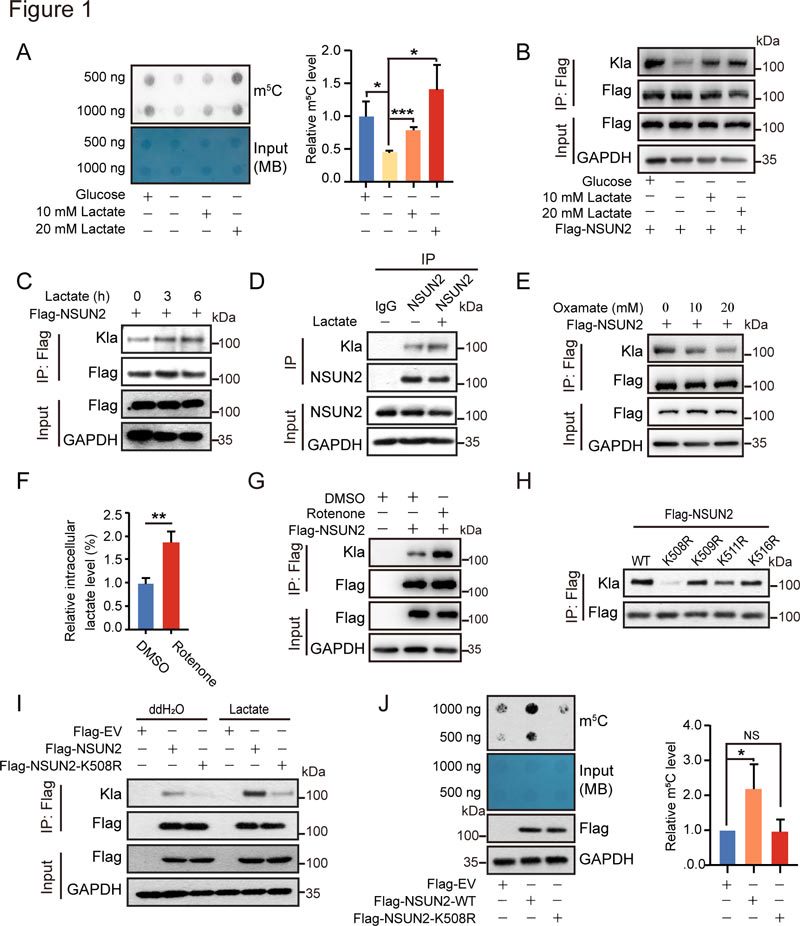
1. NSUN2 is activated and lactated by lactic acid at K508.
(A, B) HEK293 cells were transfected with Flag-NSUN2 for 48 h, followed by glucose starvation for 3 h and treatment with or without 10 or 20 mM lactic acid for 6 h. (A) Total RNA m5C level and (B) NSUN2 lactation level in immunoprecipitate. (C) The lactation level of NSUN2 was analyzed by co-IP and western blotting. (D) The lactation level of NSUN2 was detected by anti-lactate lysine antibody. (E-G) Treated with oxaline (E) or rotenone (F and G), check for NSUN2 lactation (E and G). Intracellular lactate levels (F) were measured in HEK293 cells. (H) HEK293 cells were transfected with FLAG-NSUN2-WT or Flag-NSUN2- mutations (K508R, K509R, K511R, or K516R) for 48 hours. The lactation level of NSUN2 was detected by anti-FLAG immunoprecipitation and western blot. (I) HEK293 cells transfected with Flag-NSUN2-WT or Flag-NSUN2-K508R were treated with or without 20 mM lactic acid for 24 h. The lactation level of NSUN2 was analyzed by co-IP and western blot. (J) Flag-NSUN2-WT or Flag-NSUN2-K508R were transfected into HEK293 cells for 48 hours. Total RNA m5C levels were measured by western blot analysis.
In summary, lactic acid activates the methyltransferase activity of NSUN2 by enhancing its lactate modification, thus promoting m5C modification and stabilization of GCLC mRNA, increasing intracellular GSH level, reducing lipid peroxidation, and making cancer cells resistant to iron death. In addition, NAA10 was identified as a lactate transferase of NSUN2, and its activity was significantly enhanced under lactic acid treatment.
The innovation and enlightenment of the article
The new mechanism of NSUN2 lactation was revealed
We demonstrate for the first time that the lactation of NSUN2, especially at the lysine 508 site, significantly enhances its catalytic activity in the acidic tumor microenvironment.
This modification not only improves the m5C methylation ability of NSUN2 to mRNA, but also specifically affects the stability of GCLC mRNA, thus promoting glutathione synthesis and providing cancer cells with a protective mechanism against iron death.
NAA10 was identified as lactyltransferase of NSUN2
The researchers identified and validated N-alpha-acetyltransferase 10 (NAA10) as the key enzyme responsible for adding lactate groups to NSUN2.
Explore the influence of other PTMs on NSUN2 function
Given the role of NSUN2 in a variety of biological processes, future studies could further explore whether other post-translational modifications besides lactation (such as phosphorylation, acetylation, etc.) also affect the function of NSUN2 and its substrate specificity.