Glutathione is able to link obesity and breast cancer progression through activation of mTOR signaling
Obesity is a major risk factor for poor prognosis of breast cancer, but the effect of obesity-induced tumor microenvironment (TME) metabolites on breast cancer growth and metastasis remains unclear.
On October 22, 2024, Ke Li and Zhuorong Li of the Chinese Academy of Medical Sciences published an online publication entitled "Adipocyte-derived glutathione promotes obesity-related breast cancer by Cell Metabolism (IF=27.7). regulating the SCARB2-ARF1-mTORC1 complex "research paper, which shows that fat cell-derived glutathione promotes obesity-related breast cancer by regulating the SCARB2-ARF1-mTORC1 complex.
The study performed a metabolomic analysis of TME in a mouse model of a high-fat diet (HFD) and found that glutathione (GSH) levels are elevated in TME in which obesity accelerates breast cancer.
In fat cells but not tumor cells, loss of glutamate-cysteine ligase catalytic subunits (GCLC), the rate-limiting enzyme of glutathione biosynthesis, reduced the progression of obesity-associated tumors.
Mechanistically, GSH enters tumor cells and binds directly to lysosomal global membrane protein-2 (clearance receptor Class B, member 2 [SCARB2]), interfering with the interaction between its N - and C-termini.
This, in turn, recruits mTORC1 to the lysosome via ARF1, leading to the activation of mTOR signaling.
Overall, the study demonstrates that glutathione links obesity and breast cancer progression through the activation of mTOR signaling.
Targeting the glutathione /SCARB2/mTOR axis may benefit obese patients with breast cancer.

Obesity is a major risk factor associated with several cancers Among obesity-related cancers in women, breast cancer is the leading cause of death.
Compared to women of normal weight, obese women with a high body mass index (BMI) had a higher risk of breast cancer and a higher risk of death, regardless of hormone receptor or menopausal status.
A previous study has shown that obesity causes systemic metabolic alterations and local alterations in the fat-dominated breast tumor microenvironment (TME) that promote cancer progression.
In particular, in conditions of obesity, crosstalk between breast fat cells, immune cells, and breast cancer cells plays a key role in accelerating tumor progression.
However, the exact molecular mechanism between obesity and poor breast cancer prognosis is unclear.
The behavior of cancer cells is regulated by cellular intrinsic factors as well as the availability of metabolites in TME.
Glutathione (GSH) is a tripeptide composed of glutamic acid, cysteine and glycine, which is the most abundant metabolite in cells. The increase of GSH concentration in cells and TME is associated with the occurrence, development and drug resistance of tumors.
Interestingly, glutathione is not only involved in the antioxidant defense system, but also plays a role in various metabolic processes depending on its organel-specific function.
The mammalian target of rapamycin (mTOR) is the best understood metabolite sensing and signaling pathway.
Amino acid activation of the mTORC1 complex involves two distinct pathways: Rag GTPase and ADP-ribosylation factor 1 (ARF1) via V-ATPase mediated mTORC1 lysosomal translocation.
While previous studies have shown that GSH supports mTOR activity by buffering ROS in T cells, whether GSH directly activates mTOR remains unclear.
Further research is needed on the role of glutathione in obesity-promoting breast cancer and its potential interaction with the mTOR signaling pathway.
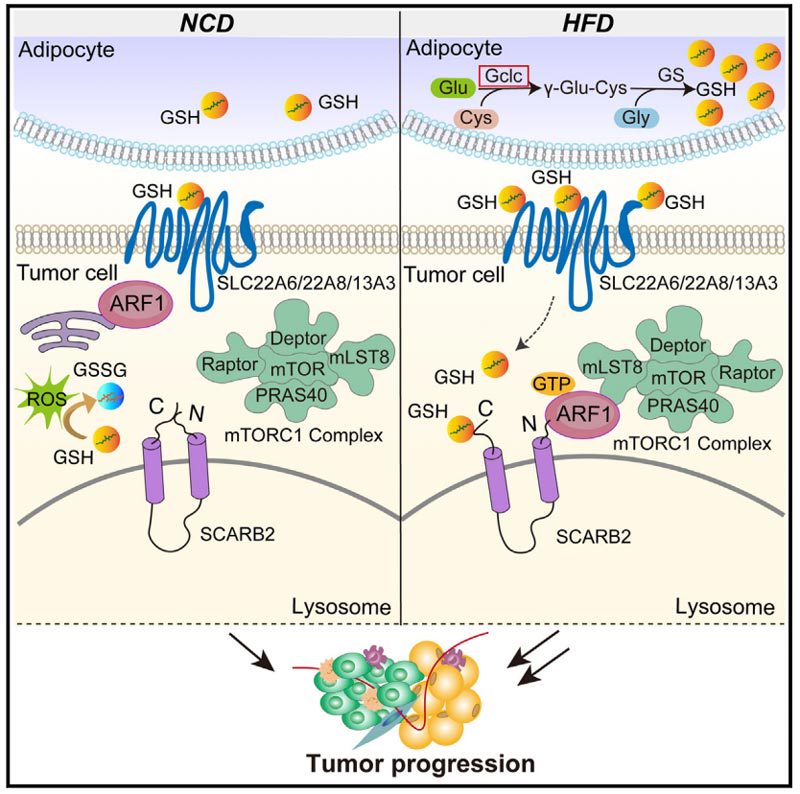
Lysosomal global membrane protein type 2 (LIMP2), also known as clearance receptor Class B, member 2 (SCARB2), exhibits different biological functions depending on the ligand with which it interacts.
The luminal domain (LD) of SCARB2 contains a cavity that promotes the binding and transport of exogenous cholesterol to lysosomal membranes and lipid droplets.
Recently, loss of SCARB2 in hepatocytes has been shown to mitigate the onset and progression of hepatocellular carcinoma (HCC) by inhibiting the self-renewal ability of cancer stem cells.
However, further research is needed to elucidate the specific role and potential mechanisms of SCARB2 in breast cancer.
Here, the researchers examined TME metabolites in the normal diet (NCD) and high-fat diet (HFD) groups, and identified the tumor-promoting effects of glutathione through in vitro and in vivo experiments.
It was found that GSH combined with SCARB2 activated mTORC1 signal.
We also explored the mechanism of GSH-induced mTOR activation, and found that the N-C-terminal interaction of GSH-interfered SCARB2 can promote the interaction between ARF1 and mammalian lethal sec-13 protein 8 (mLST8) to recruit mTORC1 lysosomal localization.
These findings provide insights into undescribed pathways linking glutathione secretion to communication between fat cells and breast cancer cells.
In conclusion, SCARB2ARF1, as a sensor of exogenous GSH, plays a crucial role in activating the mTORC1 signaling pathway.
These findings highlight the potential of targeting SCARB2 and ARF1 as therapeutic strategies, especially for obese patients, and further studies are needed to explore prognostic and therapeutic implications in more detail.