If glutathione is used in pig feed, will pork taste better?
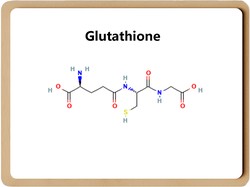
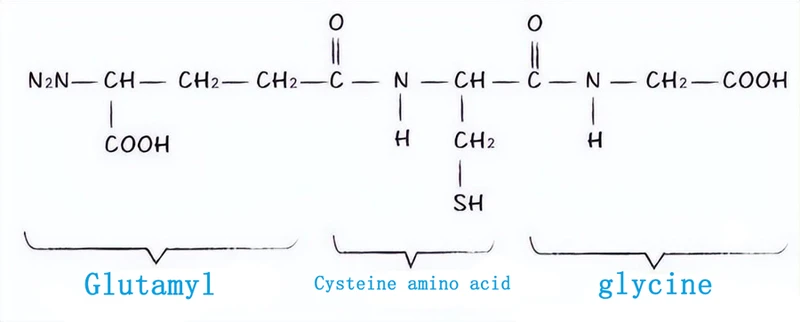
Glutathione is a tripeptide compound formed by the condensation of cysteine, glutamic acid, and glycine. It was discovered by French scientists in 1888 in the ethanol extract of bread yeast.

In 1921, it was discovered that glutathione contains glutamic acid and cysteine, so it was named "glutathione".
At present, glutathione has been widely used in the fields of food, medicine, health products, and cosmetics. There is relatively little research on glutathione as a feed additive, but it can promote healthy animal farming, improve meat quality and food safety, and has a promising market prospect.
Glutathione, as the most abundant non protein thiol, is present in all mammalian tissues. The majority of glutathione is found in the cytoplasm matrix, with 10% to 15% located in mitochondria and a small portion in the endoplasmic reticulum.
Glutathione exists in two forms: reduced and oxidized. Under physiological conditions, the majority of glutathione exists in the reduced form, accounting for 98% of the total glutathione. However, there are also several other forms of glutathione present in cells, tissues, and plasma.
GSH in the body can be oxidized to GSSG under the catalysis of glutathione peroxidase, while glutathione reductase can use reduced coenzyme II to convert GSSG to GSH, maintaining the stability of intracellular glutathione.
In addition to GSSG, GSH can also be converted into other forms of disulfide mixtures, such as forming GS-S-CoA with coenzyme A, forming GS-S Cys with cysteine, and forming GS-S protein with proteins.
The synthesis of GSH requires two steps. The first step is the coupling of glutamate cysteine ligase with ATP, where the gamma carboxyl group of glutamate forms an amide bond with the amino group of cysteine, resulting in the formation of gamma glutamylcysteine;
The second step is to synthesize tripeptide compounds through peptide bond condensation between glycine and γ - glutamylcysteine under the coupling hydrolysis catalysis of GSH synthase and ATP. GS is composed of two identical subunits and is not feedback inhibited by GSH. These reactions mainly occur in most cytoplasm, and the synthesized GSH is a widely used active short peptide.
High performance liquid chromatography is commonly used to detect the levels of glutathione and GSH.
By adding iodoacetate to modify thiol compounds, and then modifying the amino group on 1-fluoro compounds with 4-diaminobenzene, many compounds were isolated, and GSSG and GSH were identified by the movement of these compounds on HPLC.
There are many methods for industrial production of glutathione, mainly including extraction, chemical synthesis, biological fermentation, and enzymatic methods.
Extraction method is to add appropriate solvents to animal and plant tissues or yeast, and then separate and finely process them.
The chemical synthesis method is mainly composed of three raw materials: glutamic acid, cysteine, and glycine, and its production process is relatively complete.
Fermentation method mainly utilizes the metabolic products of specific microorganisms to convert carbohydrates into glutathione.
The enzymatic synthesis method generally uses GS to catalyze substrates such as glycine, L-glutamic acid, and L-cysteine in vivo, and adds an appropriate amount of ATP to synthesize glutathione.

Glutathione is the most common and important low molecular weight thiol in mammalian tissues, with various biological functions such as antioxidant, detoxification, regulation of cell proliferation and apoptosis, regulation of redox dependent cell signaling, and participation in the gamma glutamyl cycle as a continuous source of cysteine.
When glutathione is lacking in tissues, it can lead to cellular oxidation, aging, decline, pathology, or death.
Maintaining normal levels of glutathione in tissues is beneficial for preventing diseases, maintaining body health, and preventing aging and biological damage.
The antioxidant function of glutathione is mainly achieved through the reaction catalyzed by GSH Px. Mitochondria are the main site of reactive oxygen species production in the body. Excessive reactive oxygen species hinder energy synthesis in mitochondria, and lipid peroxidation reactions can cause oxidative damage to mitochondria.
GSH is oxidized by GSH Px to GSSG during the detoxification process of H2O2 or organic hydroperoxides, thereby reducing the generation of H2O2 and lipid peroxides in the body and protecting the integrity of the cell membrane.
On the contrary, GSSG is reduced back to GSH under the action of GSSG reductase, forming an oxidation-reduction cycle.
Glutathione plays a particularly important role in the defense of mitochondria against oxidative stress under physiological and pathological conditions.
GSH has a strong ability to donate electrons, and its high concentration in cells can maintain a normal cellular environment.
Compared to cysteine, GSH is less easily oxidized, making it more suitable for maintaining intracellular redox potential.
Degroote et al. found that when the ratio of GSH to GSSG increases in the red blood cells, small intestinal mucosa, and liver tissue of weaned piglets, the redox state of glutathione is enhanced.
Therefore, GSH is a very important antioxidant in the body that can protect proteins, DNA, and other biomolecules from oxidative damage.
GSH also interacts with other non enzymatic antioxidants. Vitamin E can reduce lipid hydroxyl radicals and lipid peroxides produced by polyunsaturated fatty acids, while vitamin C, GSH, selenium, etc. can reduce them to vitamin E.
Vitamin C has strong reducibility, which eliminates the toxicity of free radicals through its reducing effect. It can assist vitamin E or GSH in clearing free radicals, and also reduce vitamin E and GSSG bound to free radicals to vitamin E and GSH.
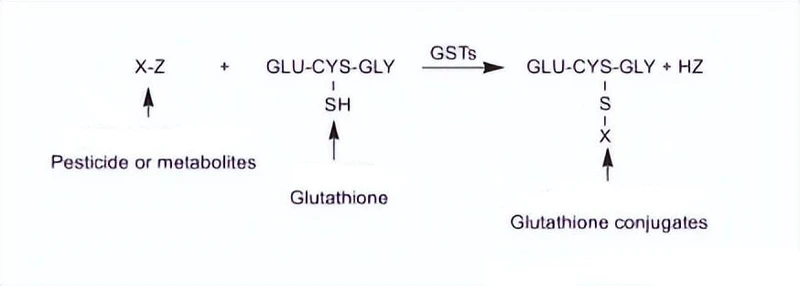
GSH binds to various harmful substances such as exogenous electrophilic reagents, toxic substances, harmful metabolites, or potential carcinogens through glutathione-s-transferase, forming more soluble and non-toxic derivatives that are secreted from cells to achieve detoxification. GSH can also act as an auxiliary factor to form the glyoxal enzyme system with glyoxal enzyme I (Glx-I) and glyoxal enzyme II (Glx-II).
With the participation of GSH, the intermediate of s-d-lactyl glutathione catalyzes the conversion of methylglyoxal (MG) to d-lactic acid, eliminating α - oxaldehyde such as MG in cells, thereby preventing the formation of α - oxaldehyde mediated ethylation products and avoiding their damage to cells.
When Glx-II catalyzes the hydrolysis of s-d-lactyl glutathione to d-lactic acid, the consumed GSH can be restored.
GSH can also bind with heavy metals in the body and be directly excreted from the body.
Tokumoto et al. found that GSH and metallothionein genes work synergistically to detoxify severe kidney damage caused by inorganic mercury.
Dong Guifang et al. found that adding an appropriate amount of GSH to feed can effectively alleviate the toxicity of microcystin toxins in yellow catfish feed, but exceeding a certain dose may have a certain inhibitory effect on fish growth.
GSH can play a role in the phagocytosis of white blood cells and the production of antibodies, and is one of the effective antiviral drugs.
GSH participates in intracellular metabolic cycles, such as the gamma glutamyl cycle. When small intestinal cells absorb and transport amino acids, the gamma glutamyl cycle is involved in the absorption of amino acids. Therefore, GSH plays an important role in the transmembrane transport of amino acids.
GSH is coupled with nitric oxide to form s-nitroso glutathione, which can be cleaved by the thioredoxin system to release GSH and NO. Endogenous NO, on the other hand, completes its targeting function under the mediation of GSH in cells.
NO and GSH are important substances for maintaining normal physiological functions of the liver. The liver can store and produce glucose, and is also the central organ of lipid metabolism in the body. It is the site of amino acid breakdown metabolism. NO and GSH play a key role in regulating lipid, glucose, and amino acid utilization by maintaining the physiological functions of the liver.
The proliferation of many normal and malignant cells is related to glutathione levels, and maintaining normal levels of glutathione in the body is extremely important for ensuring the orderly progression of the cell cycle and the entry of cells into the S phase.
Glutathione affects the members of the signal transduction chain involved in cell proliferation through S-glutathionylation, which helps maintain the levels of glutathione or thioredoxin in DNA synthesis and is necessary for maintaining the rate limiting enzyme activity of ribonucleotide reductase.
The redox state of glutathione can affect the expression and activity of many key genes involved in cell cycle activities.