Key role of adipocyte glutathione in the progression of obese breast cancer
Obesity is a major cause of many types of cancer. Among obesity-related cancers in women, breast cancer is the leading cause of death.
Previous studies have shown that obesity can induce metabolic changes in the breast tumor microenvironment (TME) and local modifications based on fat to promote cancer progression.
However, the effects of obesity-induced tumor microenvironment metabolites on the growth and metastasis of breast cancer are still unclear. Therefore, it is important to explore the crosstalk factors and mechanisms between obesity and breast cancer to improve the therapeutic effect of obese breast cancer patients.
On October 22, 2024, Ke Li and Zhuorong Li from the Institute of Medical Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College published a paper entitled Adipocyte-Derived Glutathione Promotes Obesity-Related Breast in Cell Metabolism Cancer by Regulating the SCARB2-ARF1-mTORC1 Complex.
He has long been committed to exploring the molecular mechanism of breast cancer occurrence and development. Previous studies have shown that FGD5 promotes the development and progression of basal-like breast cancer by maintaining tumor-initiating cell-like traits.
In this study, we found that high-fat diet (HFD) induces increased glutathione (GSH) secretion in adipocytes, glutathione enters tumor cells through classical intake transporters, activates mTORC1 signaling pathway through lysosomal membrane protein 2 (SCARB2) on the surface of lysosomal membrane, and promotes the progression of obesity-related breast cancer.

A mouse model of diet-induced obesity (DIO) was established to study the effect of obesity on breast cancer progression.
Compared with the normal diet group (NCD), HFD mice showed faster tumor growth, larger tumor volume and more severe lung metastasis.
The tumor microenvironment (TME) metabolites of mice in NCD and HFD groups were detected, ten metabolites with the most significant differences were selected, and the tumour-promoting effects of glutathione in metabolites were determined through in vitro and in vivo experiments.
Inhibition of glutathione production in tumor cells has no significant effect on the progression of obesity-related breast cancer, suggesting that glutathione in the tumor microenvironment may be a major factor in obesity-induced breast cancer progression.
To clarify the origin of glutathione, the authors performed multiple immunofluorescence staining on mouse tumor tissue and found that glutathione had strong aggregation in adipocytes (adiponectin positive) of HFD mouse tumor tissue.
The authors observed that fat cells from obese breast cancer patients but not tumor cells showed higher levels of glutamate-cysteine ligase (GCLC) than non-obese patients, indicating an enhanced capacity for glutathione synthesis in fat cells from obese patients.
Fat cells and tumor cells from obese breast cancer patients showed higher levels of glutathione compared to non-obese patients.
Subsequently, the authors took advantage of the endogenous expression of GCLC in preadipocytes (3T3-L1) knocked down by sgRNA and cultured breast cancer cells with the culture supernatant of the above adipocytes. It was found that adipocyte secretion could promote the proliferation and invasion of breast cancer cells, while the ability to promote the proliferation and invasion of breast cancer was weakened in the GCLC deletion group.
We established a transgenic mouse model of adipocyte specific absence of GCLC and found that impaired glutathione synthesis in adipocytes hindered the pro-breast cancer progression of HFD.
The authors hypothesized that glutathione secreted by adipocytes in the tumor microenvironment is a key factor in obesity-related breast cancer progression.
Focusing on the mechanism of glutathione promoting breast cancer progression, it was found that glutathione can enter tumor cells and locate on the surface of lysosomes.
Based on mass spectrometry (MS) proteomic analysis, RNA-seq analysis and biochemical analysis, we found that glutathione binds to lysosomal membrane surface protein SCARB2 to activate the mTORC1 signaling pathway.
We also explored the activation mechanism of mTOR induced by glutathione, and found that glutathione interferes with the interaction between the N-terminal and C-terminal of SCARB2 protein, promoting the exposure of the N-terminal of SCARB2 to bind to GTase ARF1, and promoting the interaction between ARF1 and mLST8 to recruit mTORC1 lysosomal localization. Thus activating the mTORC1 signaling pathway.
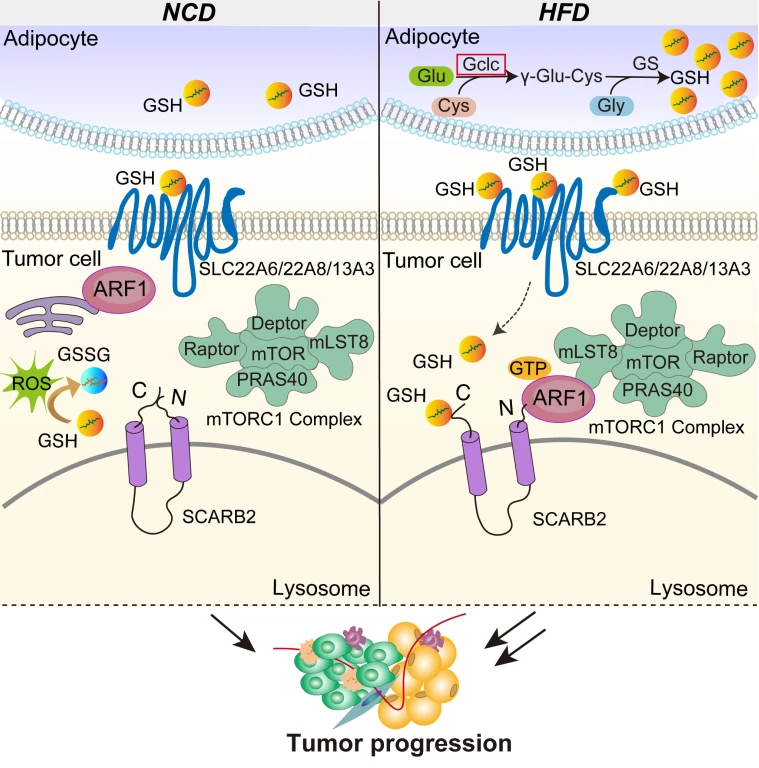
In summary, HFD leads to increased glutathione secretion in adipocytes. Exogenous glutathione enters tumor cells, binds to SCARB2 on the surface of lysosomal membrane, and activates mTORC1 signaling pathway through ScarB2-ARF1 complex, thus promoting the progression of obesity-related breast cancer.
The study revealed the role of glutathione derived from fat cells in accelerating breast cancer progression with obesity;
A novel form of mTORC1 activation mediated by glutathione was elucidated.
The role of SCARB2 as a novel glutathione sensor in regulating mTORC1 signaling pathway was proposed for the first time.
These findings highlight that targeting the GSH/SCARB2/ARF1/mTOR axis could provide a potential strategy for effective treatment of obese breast cancer patients.
reference
- 1. Lauby-Secretan, B., C. Scoccianti, D. Loomis, Y. Grosse, F. Bianchini, K. Straif, and G. International Agency for Research on Cancer Handbook Working. (2016). Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 375, 794-8. https://doi.org/10.1056/NEJMsr1606602
- 2. Reaves, D.K., E. Ginsburg, J.J. Bang, and J.M. Fleming. (2015). Persistent organic pollutants and obesity: are they potential mechanisms for breast cancer promotion? Endocr Relat Cancer. 22, R69-86. https://doi.org/10.1530/ERC-14-0411
- 3. Bray, F., J. Ferlay, I. Soerjomataram, R.L. Siegel, L.A. Torre, and A. Jemal. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68, 394-424. https://doi.org/10.3322/caac.21492
- 4. White, A.J., H.B. Nichols, P.T. Bradshaw, and D.P. Sandler. (2015). Overall and central adiposity and breast cancer risk in the Sister Study. Cancer. 121, 3700-8. https://doi.org/10.1002/cncr.29552
- 5. Sanchez-Jimenez, F., A. Perez-Perez, L. de la Cruz-Merino, and V. Sanchez-Margalet. (2019). Obesity and Breast Cancer: Role of Leptin. Front Oncol. 9, 596. https://doi.org/10.3389/fonc.2019.00596
- 6. Chenxi Zhao, T.Z., Si-tu Xue, Peitao Zhang, Feng Wang, Yunxuan Li, Ying Liu, Luyao Zhao, Jie Wu, Yechao Yan, Xiaoyun Mao, Yuping Chen, Jian Yuan, Zhuorong Li, and Ke Li. (2024). Adipocyte-derived glutathione promotes obesity-related breast cancer by regulating the SCARB2-ARF1- mTORC1 complex. Cell Metabolism. https://doi.org/https://doi.org/10.1016/j.cmet.2024.09.013
- 7. Li, K., T.T. Zhang, C.X. Zhao, F. Wang, B. Cui, Z.N. Yang, X.X. Lv, Z. Yeerjiang, Y.F. Yuan, J.M. Yu, Z.H. Wang, X.W. Zhang, J.J. Yu, S.S. Liu, S. Shang, B. Huang, F. Hua, and Z.W. Hu. (2021). Faciogenital Dysplasia 5 supports cancer stem cell traits in basal-like breast cancer by enhancing EGFR stability. Sci Transl Med. 13. https://doi.org/10.1126/scitranslmed.abb2914