- Appearance White crystals or crystalline powder
- Assay (%) ≥99.0
- CAS No.:70-18-8
- Water solubility: soluble in water, transparent
- Taste: sour, astringent, with a fresh taste similar to MSG
- 3 Factories & 9 production lines
- GMP standard workshop & two independent laboratories
- FDA Cert.
- Certifications: Halal,ISO9001,PAHS Free,NON-GMO,KOSHER,SC
- Delivery term: DHL, FEDEX, Air freight,Sea freight
- Provide Free Sample
- MOQ: 1KG
L-glutathione Powder Supplier:
GSHWORLD is a manufacture of L-Glutathione bulk powder for 20 years. Glutathione is present in the form of a white crystalline powder that can be easily dissolved in water, but is nearly insoluble in ethanol (99.5). Upon decomposition, it has a melting point of approximately 185℃. And tastes sour, astringent, with a fresh taste similar to MSG. GSHWORLD CGMP standard workshop of 2000 square meters, and a factory laboratory of 500 square meters to ensure the high qulity of products. Our products are high quality and low price, welcome to consult us.
What is glutathione?
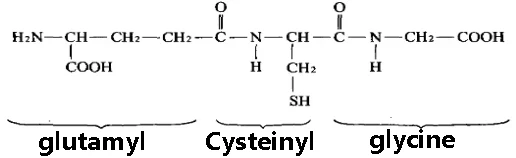
Reduced glutathione (GSH) is a tripeptide compound that is formed by the condensation of glutamate, cysteine, and glycine through a peptide bond. GSH is chemically named γ-L-glutamyl-L-cysteinyl-glycine, and its structural formula is shown in the figure provided. Unlike other peptides and proteins, It has a special peptide bond formed by the condensation of the γ-carboxyl group (-COOH) of glutamate with the α-amino group (-NH2) of cysteine.
Certificate of Analysis
Test Items | Specification | Result | |
Description | A white crystalline powder | A white crystalline powder | |
Assay (bas on dry) | 98.0%~ 101.0% | 99.0% | |
Identification | IR | Similar with the Reference Spectrum | Complies |
Optical Rotation | - 15.5°~- 17.5° | - 16.7° | |
Inorganic impurities | Chlorides | Not more than 200ppm | Complies |
Arsenic | Not more than 2ppm | Complies | |
Chlorides | Not more than 200ppm | Complies | |
Sulphates | Not more than 300ppm | Complies | |
Heavy Metals | Not more than 10ppm | Complies | |
Iron | Not more than 10ppm | Complies | |
Cadmium | Not more than 1ppm | Complies | |
Lead | Not more than 3ppm | Complies | |
Mercury | Not more than 1ppm | Complies | |
Residue on Ignition | Not more than 0. 1% | 0.06% | |
Organic impurities | Total | Not more than 2.0% | 1.3% |
GSSG | Not more than 1.5% | 0.4% | |
Clearity and color of solution | Clear and Colorless | Clear and Colorless | |
Residual solvents | Meet the requirement | Complies | |
Loss on Drying | Not more than 0.5% | 0.2% | |
Microbiology | Total plate count | ≤1000cfu/g | Complies |
Yeast and mold count | ≤100cfu/g | Complies | |
Total coliforms | ≤100cfu/g | Complies | |
E. coli | Absent/10g | Complies | |
S. aureus | Absent/10g | Complies | |
Salmonella | Absent/10g | Complies | |
Conclusion | Complies with the standard of USP 41 | ||
Purity
(1) Clarity and color of solution— Dissolve 1.0 g of Glutathione in 10 mL of water: the solution is clear and colorless.
(2) Heavy metals <1.07>— Proceed with 2.0 g of Glutathione according to Method 2, and perform the test. Prepare the control solution with 2.0 mL of Standard Lead Solution (not more than 10 ppm).
(3) Arsenic <1.11>— Prepare the test solution with 1.0 g of Glutathione according to Method 1, and perform the test (not more than 2 ppm).
Testing Assay:
Weigh accurately about 0.5g L-Glutathione Powder of the substance to be examined, previously dried, dissolve in 50 mL of a solution of metaphosphoric acid (1 in 50), and titrate with 0.05mol/L iodine VS, add 1 mL of starch indicator when the endpoint approaches. Continue titration until the solution appears blue color. Carry out a blank titration in the same manner, and make any necessary correction. 1 mL of 0.05mol/L iodine is equivalent to 30.733 mg of C10H17N3O6S.
Calculate the percentage of glutathione (C10H17N3O6S) in the portion of glutathione taken:
Result = [(V-B) X N X F X 100] / W
V =titrant volume of the sample (ml)
B = titrant volume of the blank(ml)
N = titrant normality (mEq/ml)
F = equivalency factor, 307.33 mg/mEq
W =weight of the Sample(mg)
Acceptance criteria: not less than 98.0%
Product methods:
There are two main methods to produce L-Glutathione Powder, but Guanjie has adopted a new method, the enzymatic method, the pros and cons of which are seen below.
● Chemical synthesis method
Serious pollution, high cost, basically eliminated
● Fermentation method
Mainstream method, average cost, large amount of pollution
Currently outdated
● Enzyme method
Traditional method, high cost of ATP
New method, ATP regeneration, low cost, and less pollution
Process Advantages (Compared with Fermentation Method)
● Fast reaction speed and short production cycle: The reaction is completed in 1 day, while the fermentation method of L-Glutathione Powder takes at least 5 days with no interruptions during this time.
● High yield, easy to purify:
● The amount of enzymatic reaction is 30-50 g/L, and the reaction components are determined and easy to purify. Fermentation is about 10 g/L, the components are complicated, and the purification needs to use toxic substances.
● Green and environmentally friendly, in line with national development requirements:
● No heavy metals, toxic gases, and organic substances (except alcohol used in the crystallization process) are added.
● Less energy consumption, low cost:
● ATP is renewable, and it has low raw material and labor cost.
Application:
Grade | Standard | Note |
Food grade | Food safety national standard, enterprise internal standard | Limited ions, etc., limited colonies and special bacteria |
Cosmetic grade | internal standard | Limited ions, etc. |
Pharmaceutical grade | Chinese Pharmacopoeia 2015, USP41, EP9.0, Japanese Pharmacopoeia | The most stringent European Pharmacopoeia, limiting the content of special impurities |
Health productgrade | Company internal standard | Colony |







