Maintain REDOX homeostasis to regulate corynebacterium glutamate glutathione metabolism
Corresponding author: Xu Meijuan, Jiangnan University
Published in: Chemical Engineering Journal
Published time: 2025.01
https://doi.org/10.1016/j.cej.2025.160237

Research background:
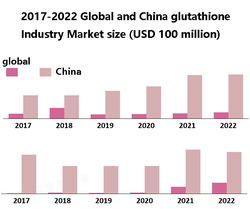
Glutathione (GSH) is a tripeptide containing L-glutamic acid, L-glycine and L-cysteine. As an endogenous antioxidant, GSH is involved in detoxification and maintenance of REDOX state, and is widely used in medicine, cosmetics and food.
Corynebacterium glutamicum is a potential host for glutathione production, but metabolic engineering strategies need to be optimized. Previous studies on maintaining cell REDOX homeostasis for efficient glutathione synthesis have been lacking, and this study aims to fill in the gaps.
Research content:
Based on the glutathione production strain G01, StGshF and StGshFE11P-H366Y-A398E (triple mutant) were introduced to obtain strains CG02 and CG03.
Under different ROS levels, the enzyme activity of mutant was significantly higher than that of control, and the highest enzyme activity reached 4.1 U/mL, which was 192.9% higher than that of wild enzyme.
Under the influence of mutant StGshFE11P-H366Y-A398E, intracellular glutathione was significantly higher than CG02, while ROS levels were not significantly reduced.
The introduction of aecD and ggt into CG03 strains resulted in the synthesis of H₂S from L-cysteine. Excessive H₂S affected cell growth. The elimination of aecD (CG04) reduced the level of H₂S and the degradation of L-cysteine, which was beneficial to glutathione synthesis.
ggt encodes gamma-glutamyl transpeptidase, decomposes glutathione to produce H₂S, knocks ggt (CG05), no H₂S is detected, and increases glutathione concentration.
Glutathione reductase (GR) was introduced, and the ScGR derived from saccharsaccharus cerevisiae was selected as the best. In CG06 strain overexpressing ScGR, intracellular and extracellular GSSG decreased, while glutathione increased.
Glutathione production was increased in CG07 strain overexpressing polyphosphokinase (PPK) mutant.
In addition, the glutathione transport system was studied. Overexpression of cg1298 and cg1299 produced CG09 and CG10 respectively, CG10 promoted the production of glutathione, and continued overexpression of cg1299 on the basis of CG10 produced CG11, which further increased the production, demonstrating that cg1299 was responsible for glutathione transport.
The glutathione yield of CG11 reached 25.07±0.41 g/L after 60 hours of fermentation in 5L tank with the strategy of adding L-cysteine in batches, controlling pH and temperature in stages and adjusting dissolved oxygen.
In SUMMARY:
In this study, Corynebacterium glutamicum was genetically engineered and the fermentation conditions were optimized to improve glutathione production efficiency by maintaining REDOX homeostasis.
Multi-target metabolic engineering strategies, including GshF modification, related gene aecD, ggt knockout, and GR overexpression, significantly increased glutathione production. After optimizing fermentation conditions in a 5 L fermenter, glutathione yield reached 25.07 g/L.
Metabolic flux analysis and REDOX status assessment revealed the mechanism of the effect of modification strategies on cell metabolism.
This study provides a valuable reference for the industrial production of glutathione, and also provides a new idea for the construction of microbial cell factories based on REDOX regulation.