Nanoliposomes containing curcumin and reduced glutathione
Liposomes are bilayer closed spherical vesicles formed from phospholipids (phosphatidylcholine, dipalmitoyl phosphatidylcholine, distearoyl phosphatidylcholine, etc.) and other components such as cholesterol and hydrophilic polymer conjugated lipids.
Drugs with very different lipophilicity can be simultaneously encapsulated in liposomes, which can be encapsulated in phospholipid bilayer, can be trapped in water, or can be encapsulated at the double-layer interface, depending on the solubility of the drug.
Studies have shown that when two drugs are delivered to cells or animals by the same carrier, their stability, bioavailability and therapeutic effect can be improved through gradual exposure and absorption.
Curcumin (CUR) is considered to be the most active component in turmeric preparations, with many physiological functions such as anti-oxidation, anti-tumor, lowering blood lipids and preventing senile dementia.
Reduced glutathione (GSH) is a tripeptide composed of cysteine, glycine and glutamic acid. Its molecular structure contains two active groups, γ-glutamyl and -SH, which exist widely in organisms and have many important physiological functions.
Through a reasonable delivery carrier design, embedding CUR and glutathione into a nanoparticle at the same time, and achieving sufficient loading capacity, may be a feasible idea to improve the stability and synergy of both.
Shaoxuan Yu, Pang Geyu, Lanlan Zhu, et AL., School of Agricultural Engineering and Food Science, Shandong University of Technology, prepared nano-liposomes (CGLIP) containing both CUR and glutathione. The polysaccharide modified CGLIP (ALCH-CGLIP) was obtained by combining CH and Al layer by layer on the surface of the LIP. The embedding rate, slow release, particle size, surface potential, micro-morphology, chemical composition and thermal stability of LIP were characterized by various spectral methods and electron microscopy, and the potential of LIP as a cooperative transport carrier of CUR and glutathione was investigated. In order to provide a certain theoretical reference and technical support for the development of new functional foods or drugs with CUR and glutathione or other bioactive substances.

Embedding rates of CUR and glutathione in 9 LIP samples
The embedding rate is one of the important indexes to evaluate the drug carrying capacity of LIP.
Because CUR was not detected in the precipitation and collection tube filtrate, and the amount of CUR in the liposomes extracted by chloroform was basically the same as the amount of CUR added during preparation, the embedding rate of CUR obtained in this study was 100%. In addition, the results of reverse rotation of the filter after inversion are consistent with these results.
Coembedding with GSH also had no significant effect on the embedding rate of CUR, which may be due to the fact that the 4 mg CUR added during the preparation of both single-embedding and coembedding LIP was either embedded in liposomes or embedded on the phospholipid bilayer.
Compared with the results reported in the literature (41% ~ 90%), the embedding rate of CUR in this study is slightly higher, which may be due to the difference in the chemical composition and structure of phospholipids, or the difference in the mass ratio of CUR to the main components of liposomes such as soybean lecithin and cholesterol.
The results showed that there was no significant difference in the embedding rate of CUR in the liposomes after monolayer CH modification and bilayer AL-CH modification, and it was still close to 100%.
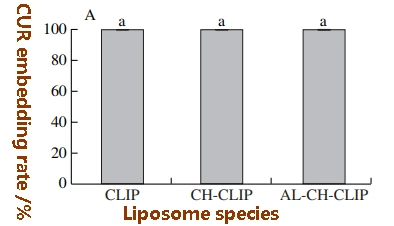
Similar to CUR, the glutathione embedding rates determined by the two methods were basically the same. However, coembedding with CUR and polysaccharide modification had a significant effect on the inclusion rate of glutathione in liposomes.
The glutathione embedding rate of liposomes increased with the increase of the number of polysaccharide modification layers (P < 0.05). In single-coated glutathione liposomes, glutathione inclusion first increased from 7.90% (GLIP) to 16.72% (CH-GLIP) and then to 37.52% (AL-CH-GLIP). In the CUR glutathione co-embedded liposomes, glutathione inclusion first increased from 27.03% (CGLIP) to 32.09% (CH-CGLIP) and then to 41.22% (AL-CH-CGLIP). There are two possible reasons for the significant increase in glutathione embedding rate of liposomes in both systems: First, CH and AL embedded partially free glutathione in the solution during the process of self-assembly to the surface of the liposome. Secondly, the polysaccharide modified liposomes are more stable, which reduces the loss of glutathione in the process of washing and purification. In the case of CUR and glutathione co-embedding, the embedding rate of glutathione in the unmodified, CH monolayer and AL-CH double-modified liposomes was 4, 2 and 1.2 times higher than that in the single encapsulated glutathione liposomes, respectively. The inclusion of hydrophobic CUR and hydrophilic substance in liposomes at the same time may improve the inclusion ability of hydrophilic substance, and this phenomenon can be explained by the effect of CUR on the structure of phospholipid bilayer. Studies have shown that CUR can be inserted deep into the lipid bilayer in a cross-bilayer direction and fixed by forming hydrogen bonds with the lipid phosphate groups, and the insertion of CUR can tighten the acyl chain region of the liposome, reduce the permeability of the liposome membrane, and reduce glutathione leakage during preparation, centrifugation, washing and other processes. The effect of polysaccharide modification on glutathione inclusion rate may be much greater than that of co-embedding with CUR, so the difference in glutathione inclusion rate between AL-CH-CGLIP and AL-CH-GLIP is not significant.Average particle size, PDI and Zeta potential analysis
The average particle sizes of BLIP, CLIP, GLIP and CGLIP were (95.02±1.93), (87.30±0.91), (117.77±1.00) nm and (132.47±18.14) nm, respectively. PDI was 0.200±0.023, 0.146±0.007, 0.184±0.008 and 0.317±0.011, respectively. The Zeta potentials were (-22.47±1.96), (-24.80±0.29), (-15.67±0.67) mV and (-14.70±0.46) mV, respectively.
The PDI of the four LIP solutions were all small (less than 0.35), indicating that their particle size was relatively uniform. Compared with BLIP, the average particle size of CLIP was reduced by 7.7 nm, while the Zeta potential did not change significantly.
The high conjugated structure of the CUR molecule makes it have strong lipophilic properties. During the formation of bilayer in soybean lecithin, CUR may be deeply inserted into the interior of the bilayer, inducing the acyl chain region of the liposome to tighten up, rather than adsorb on the surface of the liposome, resulting in a slight reduction in CLIP particle size without significant change in surface charge.
The mean particle size of GLIP increased by about 13 nm, and the Zeta potential increased by about 7 mV, indicating that glutathione was embedded in the aqueous cavity of liposome, and some glutathione may be adsorbed on the surface of liposome. Glutathione is dissolved in PBS at pH 6, the pH is close to the isoelectric point of glutathione (5.93), and glutathione itself has only a weak negative charge.
Glutathione is not only embedded in the water phase cavity inside the liposome, but also adsorbated on the surface of the liposome through the formation of hydrogen bonds between amino and phosphate groups or van der Waals forces, which can be confirmed by FTIR analysis results.
Compared with BLIP, the mean particle size of CGLIP increased by 37.45 nm, and the Zeta potential increased by about 8 mV, which may be because more glutathione was embedded in the liposomes when glutathione was co-embedded with CUR, and some glutathione was absorbed on the surface of the liposomes, which was consistent with the results of the previous embedding rate analysis.
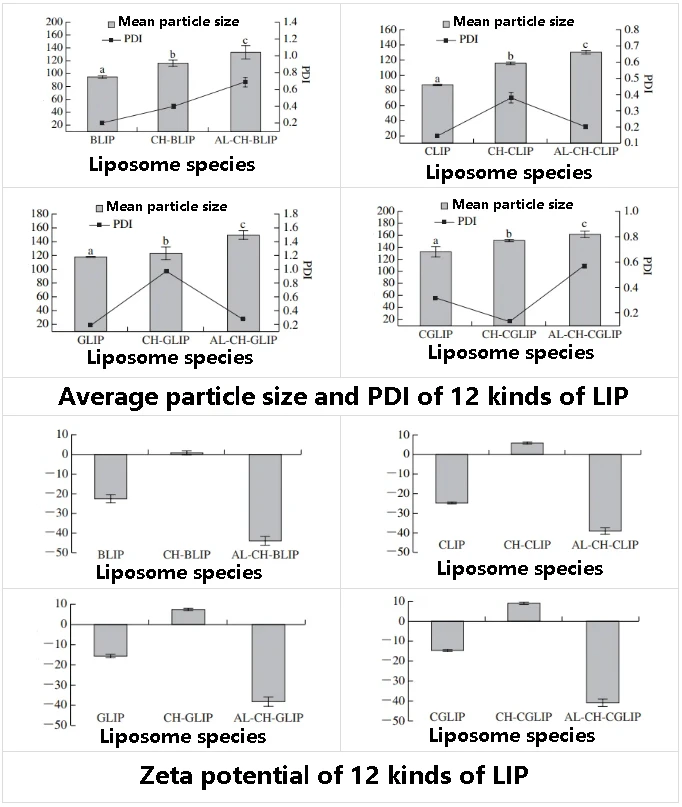
The average particle sizes of CH-BLIP, CH-CLIP, CH-GLIP and CH-CGLIP were (116.37±4.80), (115.93±1.60), (123.33±1 8.04) nm and (151.90±1.80) nm, respectively. The Zeta potentials were (0.77±1.08), (5.98±0.36), (7.49±0.49) mV and (9.00±0.68) mV, respectively. After the AL-CH double layer modification, The average particle sizes of AL-CH-BLIP, AL-CH-CLIP, AL-CH-GLIP and AL-CH-CGLIP are (133.23±16.26), (130.50±1.93), (149.87±12.25) nm and (161.97±5.58) nm, respectively. The Zeta potentials were (-43.93±2.08), (-39.07±1.64), (-38.23±2.41) mV and (-40.87±1.79) mV, respectively.
The Zeta potential of the liposomes changed from negative to positive first and then from positive to negative after polysaccharide modification, indicating that CH and AL self-assembled to the surface of the liposomes in turn through electrostatic interaction. The results are consistent with those previously reported by our research group.
With the increase of the number of polysaccharide modification layers, the particle size of the liposome gradually increased (P < 0.05), and the PDI also increased significantly. Especially after the CH modification, the maximum PDI of the liposome was about 0.9, which may be because the absolute value of the Zeta potential of the liposome was the smallest at this time, resulting in the worst stability and the easy cross-linking between particles, resulting in uneven particle size distribution.
Although the uniformity of LIP size distribution was reduced, this result once again proved that CH and AL modified the surface of the liposomes, and the Al-CH bilayer modified liposomes had stronger stability than the CH monolayer modified liposomes.
In vitro release rates of CUR and/or glutathione from different LIP
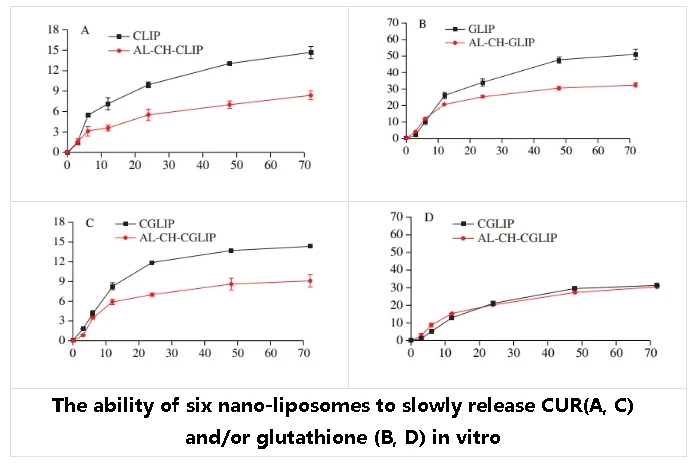
All types of nanoliposomes have the effect of slowly releasing CUR or/and glutathione. However, the release rate of CUR and glutathione from the nanoliposomes modified by AL-CH is smaller and has better sustained release.
After 72 h of dialysis, the release rates of AL-CH-CLIP and CLIP to CUR were 8.4% and 14.6%, respectively, and the release rates of AL-CH-GLIP and GLIP to glutathione were 32.7% and 51.2%, respectively. AL-CH-CGLIP and CGLIP had 9.1% and 13.4% release rates for CUR and 30.6% and 31.4% release rates for glutathione, respectively.
AL-CH bilayer modified nanoliposomes showed better sustained release of CUR and glutathione than unmodified nanoliposomes.
This may be due to the strong electrostatic interaction between AL, CH and phospholipid membrane pairs, which increases the colloidal stability of the liposome nanoparticles, thereby slowing down the release rate of CUR and glutathione. This is consistent with the results of Yu Shaoxuan and Zhu Yuqing et al.
When the CUR and glutathione were co-embedded, the nano liposomes had no significant effect on the release rate of CUR, but the release rate of glutathione was reduced compared with the CUR or glutathione single-embedded nano liposomes.
This may be because the embedding of CUR can increase the order and rigidity of phospholipid bilayer hydrocarbon chain accumulation, and improve the stability of nano liposomes.
AL-CHGLIP had a higher glutathione release rate than GLIP in the first 6 h, and AL-CH-CGLIP had a higher glutathione release rate than CGLIP in the first 12 h, which may be caused by the embedding of glutathione in the process of polysaccharide recombination to the surface of liposomes.
This part of glutathione is adsorbed on the surface of the liposome and is easily released, while the glutathione wrapped in the inside of the liposome is difficult to release.
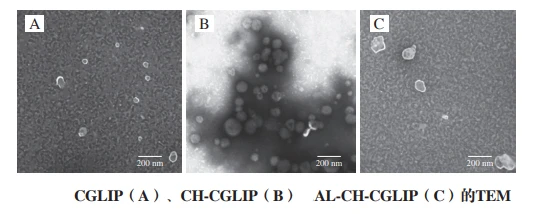
The micromorphology of different nano-liposomes
CGLIP is a regular spherical particle, well dispersed in the field of view, the core is hollow, the shell is white bright bilayer, the particle size varies in the range of 40 ~ 100 nm.
CH-CGLIP is also a relatively regular spherical particle with a size distribution in the range of 75 ~ 155 nm. However, there is a relatively obvious aggregation among CH-CGLIP particles, which may be related to the relatively small content of surface charge, which is consistent with the previous Zeta potential and PDI measurement results.
Moreover, there are strong staining traces around CH-CGLIP, which may be caused by phosphotungstic acid binding with positively charged CH ions. Phosphotungstic acid is an anionic stain that binds to positively charged parts of the lipid structure.
The shape of AL-CH-CGLIP becomes irregular, with a particle size distribution between 90 and 180 nm. Compared with CGLIP, the particle sizes of CH-CGLIP and AL-CH-CGLIP are significantly increased, which is consistent with the results of particle size analyzer.
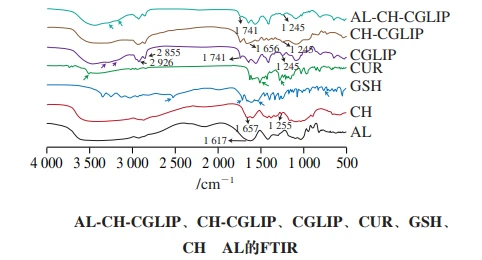
Composition and interaction of nano-liposomes
The presence of phospholipids can be characterized by the appearance of several functional group characteristic peaks:
The characteristic peaks of symmetric and asymmetric CH2 stretching vibration in the range of 3 000 ~ 2 800 cm-1, the characteristic peaks of C = O stretching vibration in the range of 1 765 ~ 1 720 cm-1, The characteristic peaks in the range of 1 200 ~ 1 145 cm-1 are due to P = O symmetric vibration, and the characteristic peaks in the range of 1 145 ~ 970 cm-1 are due to P-O-C symmetric vibration.
In the spectrum of CGLIP, the symmetric and asymmetric stretching vibration characteristic peaks of CH2 appear at 2 926 cm-1 and 2 855 cm-1, the stretching vibration characteristic peak of C = O appears at 1 741 cm-1, but the symmetric vibration characteristic peak of P = O appears at 1 245 cm-1, with a blue shift. This may be due to the presence of CUR and glutathione.
However, many characteristic peaks of CUR do not appear in the spectrum of CGLIP, such as the characteristic peak of phenolic hydroxyl stretching vibration (at 3 515 cm-1), the characteristic peak of C = C ring and C = O vibration overlapping (at 1 508 cm-1), and the characteristic peak of C-O vibration in the enol structure (at 1 270 cm-1). The characteristic peak intensity at 1 185, 1 119, 1 154 cm-1 and 1 026 cm-1 also decreased significantly, indicating that the CUR was mainly embedded in the bilayer of the liposomes, and very few free CUR might be adsorbed on the surface of the liposomes.
Many characteristic peaks of glutathione were also not observed in the spectrum of CGLIP, but multiple absorption peaks appeared at 3 400 to 2 700 cm-1 due to O-H and N-H stretching vibrations and their overlapping, and the intensity was significantly weakened, indicating that glutathione was mainly embedded in liposomes. Some of the free glutathione may be adsorbed on the surface of liposomes.
By comparing the spectra of CH-CGLIP, ALCH-CGLIP and CGLIP, it can be found that the position of the characteristic peaks generated by symmetric and asymmetric CH2 stretching vibration and P = O symmetric vibration of soybean lecithin basically remain unchanged, but the peak intensity decreases significantly, preliminatively demonstrating that CH and AL cover the surface of liposomes.
The characteristic peak of CH was observed in the spectrum of CH-CGLIP (1 656 cm-1) in the amide I band, and the position of the peak was slightly shifted.
Combined with the shift and intensity transformation of the characteristic peaks of the preceding phosphate groups, it is further proved that CH is successfully modified on the surface of the liposome through electrostatic interaction.
The characteristic peak representing C-O at 1 255 cm-1 did not appear in CH-CGLIP, and the characteristic peak at 3 500 cm-1 showed a redshift, indicating that CH may also bind to phospholipids in liposomes through the formation of intermolecular hydrogen bonds.
In Al-CH-CGLIP, the characteristic peak of the amide I band of CH and the characteristic peak of the carboxyl group of AL (at 1 617 cm-1) did not appear, indicating that AL was mainly bound to CH through electrostatic interaction and modified on the nano-liposomes, which is consistent with the previously reported results.
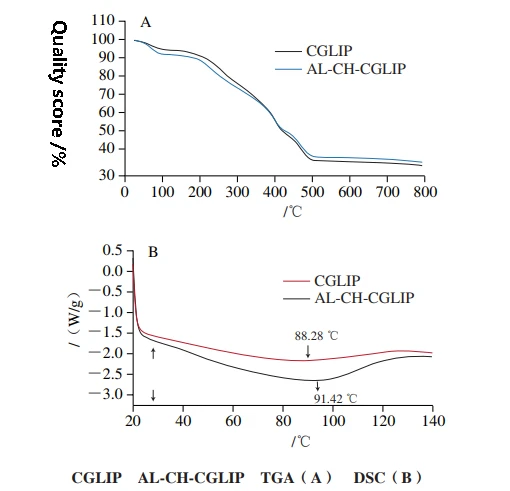
Thermal stability of CGLIP and AL-CH-CGLIP
The mass loss of CGLIP and AL-CH-CGLIP can be roughly divided into 3 stages. The mass loss rates of CGLIP and ALCH-CGLIP are 4.62% and 7.08%, respectively, in the range of 20 ~ 150 ℃.
Since the mass loss in this temperature range is mainly caused by water evaporation, the results show that more water is lost in the heating process of Al-CH-CGlip than in CGLIP, which may be because functional groups such as -- OH, -- COOH and -- CONH in the molecular structure of AL and CH bind more water.
In the range of 150 ~ 500 ℃, the mass loss rates of CGLIP and Al-CH-CGLIP were 56.95% and 52.02%, respectively, which was mainly caused by the decomposition of lecithin, cholesterol, CUR, glutathione, CH and AL, the main components of nano-liposomes. The results show that the decomposition degree of CGLIP is slightly higher than that of AL-CH-CGLIP during heating.
At temperatures above 500 ℃, both CGLIP and AL-CH-CGLIP show slow mass loss, which is caused by sample carbonization.
In general, the mass loss of CH-AL modified liposomes during heating is significantly lower than that of unmodified liposomes, indicating that CH-AL double-layer modification can increase the thermal stability of liposomes to a certain extent.
Generally, the DSC curve of macromolecular substances does not appear sharp peaks, but only slow and smooth peaks. The DSC curves of CGLIP and ALCH-CGLIP showed a wide endothermic peak, and the peak value was 88.42 ℃ and 91.42 ℃, respectively.
Compared with previously reported results, the temperature of endothermic peaks in CGLIP was slightly higher than that in empty liposomes and single-encapsulated CUR liposomes, indicating that coembedding of CUR and glutathione may increase the thermal stability of nanoliposomes under such conditions, which is similar to the results of Hou Lifen et al.
Compared with CGLIP, the endothermic peak of Al-CH-CGlip is shifted in the direction of temperature increase, which indicates that the presence of AL-CH in the outer layer of Al-CH-CGlip can enhance their resistance to temperature, consistent with the results of TGA.
Liu Yujia et al. also found that CH assembled on the surface of CUR liposomes could protect the liposomes and reduce CUR leakage under high temperature conditions.
Concluding discussion
In this study, nano-liposomes containing CUR and glutathione were prepared, and CH and AL were modified to the surface of the nano-liposomes by electrostatic interaction to enhance the stability of the nano-liposomes.
The results showed that co-embedding with glutathione, CH monolayer modification or AL-CH bilayer modification had no significant effect on the embedding rate of CUR.
However, when co-embedding with CUR, the inclusion rate of glutathione increased from 7.90% (GLIP) to 27.03% (CGLIP), and after polysaccharide modification, the inclusion rate of glutathione further increased to 32.09% (CH-CGLIP) and 41.22% (AL-CH-CGLIP).
Compared with BLIP, CLIP and GLIP, the mean particle size and Zeta potential of CGLIP are slightly increased.
With the increase of the number of polysaccharide modified layers, the average particle size of liposomes also increased gradually, but compared with the CH monolayer modified liposomes, the absolute Zeta potential of AL-CH bilayer modified liposomes was larger and had higher dispersion stability.
Under the condition of oscillating incubation at 37 ℃, the release rates of both CGLIP and AL-CH-LIP to CUR were significantly higher than those of glutathione, and the sustained release effects of ALCH-CGLIP on CUR and glutathione were better than those of CGLIP.
The denaturation temperature of al-CH-CGLIP during heating is slightly higher than that of unmodified liposomes, but the mass loss rate is significantly lower than that of unmodified liposomes, indicating that AL-CH double-layer modification can increase the thermal stability of liposomes to a certain extent.
This study elucidates the effects of CUR and glutathione co-embedding and AL-CH double-layer modification on the embedding rate, slow release and stability of nano-liposomes, which can provide certain reference value for the development of novel functional foods or drugs containing hydrophobic and hydrophilic active substances at the same time.