Role of glutathione peroxidase 4 iron death defense system in treatment of triple-negative breast cancer
Abstract
Iron death is a mode of cell death with lipid peroxides as the core, and cells can rely on the glutathione peroxidase 4 (GPX4)/glutathione (GSH) antioxidant system to reduce iron death sensitivity.
Triple negative breast cancer (TNBC) cells are more dependent on the intracellular antioxidant mechanism than normal cells, and the induction of iron death based on the GPX4/ glutathione system shows significant promise for TNBC treatment.
This article reviews the recent research results of iron death treatment of TNBC based on GPX4/ glutathione, in order to provide reference for the clinical treatment of TNBC.
keyword
Iron death; Triple negative breast cancer; Glutathione; Glutathione peroxidase 4
According to a report by the World Health Organization in February 2024, breast cancer is the cancer with the highest diagnosis rate and incidence among women worldwide.
According to the estrogen receptor (ER), progesterone receptor (progesterone receptor) on the surface of breast cancer cells, PR) and human epidermal growth factor receptor 2 (HER-2) were typed, and all three receptors were negative for triple negative breast cancer (TNBC).
TNBC is highly invasive, has a poor prognosis, poor efficacy of targeted or hormonal therapy, and lacks effective targeted treatment.
Iron death is caused by the production of a large amount of reactive oxygen species (ROS) in cells under iron dependence, and the reaction of ROS with lipids leads to the accumulation of a large amount of lipid reactive oxygen species (Lip-ROS). The type of cell death that causes cell rupture and death.
Iron death is double-sided in tumorigenesis:
On the one hand, the occurrence of iron death can effectively prevent the development of cancer;
On the other hand, the inflammatory response and immunosuppression caused by iron death are conducive to tumor growth.
It was found that by down-regulating the defense pathway to promote iron death in TNBC cells, tumor growth could be inhibited.
Multiple omics sequencing by YANG et al. found that TNBC luminal androgen receptor (LAR) tumor was significantly correlated with iron death, mainly due to iron death inhibition pathway and glutathione peroxidase 4, GPX4)/glutathione (GSH).
ferroptosis
In the 19th century, Fenton H J discovered the existence of Fenton reaction in cells, that is, iron divalent ions react with peroxide to produce hydroxyl radicals.
Cells have a corresponding scavenging mechanism for free radicals and their derivatives produced by Fenton reaction, and inhibition of such scavenging mechanism may become a new programmed cell death mode.
Based on this, Dr.Brent found a new compound, glutathione peroxidase 4 inhibitor (RAS-selective lethal 3, RSL3). Inhibition of glutathione peroxidase 4 induced an iron-dependent cell death different from apoptosis, which was named as iron death.
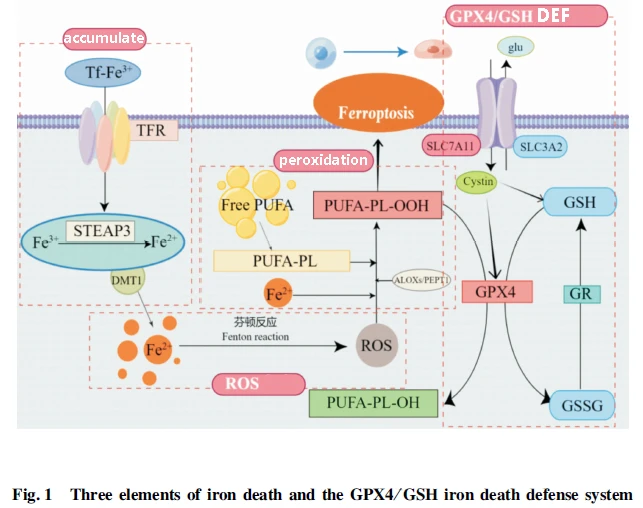
Iron death occurs with three elements: iron accumulation, ROS production, and lipid peroxidation:
Iron accumulation mainly depends on transferrin (TF) to transport trivalent iron into the cell in the form of internal vesicles via the transferrin receptor (TFR). six-transmembrane epithelial antigen of the prostate 3 (STEAP3) reduces trivalent iron to bivalent in endocytotic vesiculus. Iron bivalent is released into the cytoplasm via the bivalent metal ion transporter 1 on the endocytosis vesicle to complete iron accumulation.
The bivalent iron provided by iron accumulation reacts with intracellular peroxides to produce ROS, and ROS provides substrate for subsequent lipid peroxidation.
The peroxidation of polyunsaturated fatty acids (PUFA-phospholipid (PUFA-PL) under the action of ROS is the key step in the death of iron.
As an iron-dead lipid substrate, PUFA-PL is the product of the co-action of acetyl-CoA by A member of the long chain family of acetyl-CoA synthetase 4 and lysophosphatidyl acyltransferase 3.
The lipid peroxidation of PUFA-PL with the participation of ROS produces PUFA-phospholipid-hydroperoxide (PUFA-PL-OOH), which can rupture the cell membrane and eventually lead to cell death.
The main cellular defense against iron death is the clearance of lipid peroxides, which are currently known to rely on glutathione, Coenzyme Q and tetrahydrobiopterin.
The glutathione peroxidase 4/ glutathione system is the earliest defense system that has been comprehensively studied and widely applied, and it mainly relies on the cystine/glutamate antiporter system, System-Xc tranports the precursor of GSH and GPX4's functional amino acid cysteine into the cell, and then the synthesized glutathione works with glutathione peroxidase 4 to clear Lip-ROS.
It was found that the suppression of System-XC-related functional subunit SLC7A11 [System-Xc is composed of heavy chain subunit solute carrier family 3 member 2, Expression of SLC3A2) and light chain subunit solute carrier family 7 member 11 (SLC7A11) can lead to GSH depletion or inhibit or even inactivate glutathione peroxidase 4, ultimately leading to lipid peroxide accumulation. Sensitize cells to iron death.
Based on this mechanism, different drugs can be developed to induce iron death by depleting GSH.
Iron Death with TNBC
Tumor cells have rapid proliferation and metabolism, and the processes of intracellular amino acid metabolism and lipid metabolism are different from those of normal cells, and more dependent on the intracellular antioxidant mechanism.
The glutathione peroxidase 4/GSH pathway is an important intracellular antioxidant mechanism and an important part of the iron death defense system.
GOUT et al. found that drug targeted inhibition of System-Xc to induce cell iron death can play an anti-Hodgkin lymphoma role.
YANG et al. showed that covalent binding of selenocysteine at the nucleophilic active site of glutathione peroxidase 4 to RSL3 can cause GPX4 inactivation and inhibit the growth of fibrosarcoma in mice.
LIU et al. designed two photodegradation-targeting chimeras (PDTACs), which can selectively degrade GPX4 and cell lysates in living cells under red light irradiation, and promote iron death in tumor cells.
Based on the dependence of tumor cells on the antioxidant System of glutathione peroxidase 4/GSH cells, some drugs acting on nodes such as System-XC and GPX4 may have an important effect on tumor cells by inducing iron death.
Iron death plays an important role in the occurrence and development of TNBC. Wang Yongxia et al. have shown that inhibiting the activation of glutathione peroxidase 4/GSH iron death defense pathway can induce iron death of breast cancer cells, thereby inhibiting tumor development.
Glutathione peroxidase 4 ubiquitination or cystine deficiency can inhibit this defense pathway and induce iron death.
CHEN et al. proved that TNBC cells are highly sensitive to Cys deficiency, and Cys deficiency will block GSH biosynthesis and increase intracellular ROS level, thereby inducing iron death in TNBC cells, which may be an effective anticancer strategy to prevent the development of TNBC.
DING et al. found that the combination of glutathione peroxidase 4 inhibitor feverolide derivatives with GPX4 could lead to ubiquitination of GPX4 and induce iron death in TNBC cells, and proposed that this mechanism could be used as a new idea to induce iron death.
ZHANG et al. found that upregulation of whole lactoferrin could lead to increased ROS in TNBC cell line MDA-MB-231 and induce iron death, while non-TNBC cell line MCF-7 was resistant to whole-lactoferrin-induced iron death.
YANG et al. also found a higher ratio of GSH and glutathione oxidized (GSSG) in the LAR subtype of TNBC, and the LAR subtype had a higher sensitivity to GPX4 inhibitors, providing theoretical support for the feasibility of iron death treatment of TNBC.
As a tumor suppressor gene, p53 can induce apoptosis and senescence by blocking cell cycle and play an anti-tumor role.
p53 can induce iron death by interfering with the glutathione peroxidase 4/GSH iron death defense system, thereby inhibiting the proliferation and migration of TNBC cancer cells.
Li Bing et al. found that the positive expression rate of p53 in cancer tissues of TNBC patients was higher than that of non-TNBC patients. WEI et al. found that amforomycin could ubiquitinate mutated p53 and induce apoptosis and iron death.
DARB-ESFAHANI et al. found that the mutation rate of p53 in TNBC was higher than that of other subtypes of breast cancer.
JIANG et al. showed that the p53 acetylation-deficient mutant p53KR lost the ability to block cell cycle, induce apoptosis and cell senescence, but retained the function of inhibiting SLC7A11 expression, thereby reducing Cys intake, interfering with the glutathione peroxidase 4/ GSH iron death defense system and inducing iron death in TNBC cells.
In addition to p53, there are other genes that inhibit breast cancer, such as MicrornA-324-3p and MicrornA-382-5P that inhibit breast cancer by inducing iron death by binding to the 3 '-UTR of glutathione peroxidase 4 and SLC7A11.
TNBC therapeutics based on GPX4/GSH iron death defense system
Iron death plays an important role in the treatment of TNBC.
Progress has been made in the study of drugs that can induce iron death in TNBC cells by interfering with the glutathione peroxidase 4/GSH iron death defense system, and drugs such as Erastin and artemisinin (Art) have been or will be put into clinical use.
At present, the focus is to develop drugs that can act on TNBC cells with high selectivity, so as to achieve the purpose of reducing toxic side effects and efficient utilization.
Erastin@FA-exo
Erastin, as a low molecular weight chemotherapy agent, can exert anti-tumor effects by inhibiting System-Xc activity.
CHEN et al. found that Erastin can significantly inhibit the viability of breast cancer cells, but Erastin is limited by low water solubility and renal toxicity, requiring a highly efficient drug delivery vector with low side effects.
YU et al. loaded Erastin into folic acid (FA) -labeled exosomes by ultrasound, and constructed an in vitro drug delivery agent for TNBC Erastin@FA-exo, Erastin@FA-exo, which could specifically target FA receptor overexpression TNBC cells;
YU et al found that Erastin@FA-exo can actively and selectively target MDA-MB-231 cells, more effectively transport Erastin into cells, inhibit the expression of glutathione peroxidase 4, cause ROS overproduction and GSH depletion, and have a higher inhibition rate on TNBC cell proliferation and transfer.
TaFe-Zif-Art
As a natural medicine, peroxy bridge in the chemical bond of Art can break after binding with intracellular ferrous ions, producing high cytotoxic ROS, promoting cell apoptosis and playing an anti-tumor role.
Art itself has poor water solubility, weak cytotoxicity, and limited intracellular iron content, so its clinical application is limited.
Lizi Haoran combined iron rich metal material to construct Art iron ion composite nanoparticle TaFe-Zif-Art. Compared with single drug, TaFe-Zif-Art can increase ROS level in MDA-MB-231 cells more effectively, resulting in a large consumption of glutathione and a downregulation of glutathione peroxidase 4 expression. Therefore, TaFe-Zif-Art is expected to provide a new idea for the treatment of TNBC.
Based on this idea, finding or designing suitable iron death induced drug delivery vector may improve the specificity of drugs to TNBC, reduce the side effects, and thus improve the efficacy.
anomanolide C, AC
AC is a natural nightshade lactone extracted from the Chinese herbal medicine Dragon ball, and many studies have shown that it has strong anti-tumor activity.
CHEN et al. found that AC causes Fe2+ accumulation through ubiquitination of GPX4, thus inducing iron death and inhibiting the proliferation and migration of TNBC.
Based on this, continue to study and reduce the toxic and side effects of AC, and develop AC vector with high selection for TNBC, AC may become a feasible scheme for future chemotherapy of TNBC.
Fe3O4@PCBMA-SIM
Statins are primarily used clinically to control hypercholesterolemia, but recent research suggests they have a potential role in cancer prevention.
simvastatin (SIM), as A 3-hydroxy- 3-methyl-glutaryl coenzyme A reductase (3-Hydroxy -3-methyl glutaryl coenzyme A reductase (HMGCR) inhibitor, Mevalonate pathway and GPX4 can be inhibited by inhibiting HMGCR.
Based on the inhibitory effect of SIM on GPX4, YAO et al. loaded SIM onto magnetic nanoparticles Fe3O4@PCBMA to prepare a new nanomedicine Fe3O4@PCBMA-SIM, and administered the drug to MDA-MB-231 and MCF-7 cell lines. The results showed that, The cytotoxicity of Fe3O4@PCBMA-SIM to MDA-MB-231 was stronger than that of MCF-7, indicating that SIM could effectively inhibit the proliferation of TNBC.
Other drugs
salazosulfapyridine (SASP) is a SLC7A11-mediated System-Xc inhibitor.
TIMMERMAN et al. pointed out that the combination of SASP and carboplatin has a significant effect on the treatment of TNBC, and recommended its clinical application.
YU et al. found that the expression of SLC7A11 and GPX4 in MDA-MB-231 cells decreased with the increase of SASP level, and SASP could induce iron death caused by SLC7A11 inhibition and ROS accumulation.
Some studies have found that zonin can induce iron death in TNBC.
Xin Li et al. found that bilonin can promote the expression of p53 and inhibit the expression of SLC7A11 and glutathione peroxidase 4, thus promoting the iron death of TNBC cells.
As an inhibitor of the rate-limiting enzyme gamma-GCs in the GSH synthesis pathway, buthionine-sulfoxylamine can directly hinder the synthesis of GSH, resulting in the inactivation of glutathione peroxidase 4 and the accumulation of ROS.
conclusion
Because of its rapid proliferation and rapid metabolism, tumor cells are more dependent on the intracellular antioxidant mechanism than normal cells, which is the key to cell defense against iron death.
The cellular antioxidant mechanism composed of glutathione peroxidase 4/GSH is an important defense mechanism for iron death.
Iron death plays an important role in the treatment of TNBC. There has been progress in the study of drugs related to inducing iron death in TNBC cells by interfering with the glutathione peroxidase 4/GSH iron death defense system, such as Erastin@FA-exo, TaFe-Zif-Art and salazosulapyridine.












